The AEMPS takes part on the STARS Project, one of the European Innovation Network (EU-IN) projects, funded by the European Commission. The Project aims to bridge the regulatory knowledge gap in academic research and to improve the direct regulatory impact of the results obtained in academic medical research.
It is observed that there is often a lack of specific relevant knowledge in regulatory science in academia that delays the development of new treatment strategies or limits the chances that promising innovations will reach patients. Therefore, early exchange and communication between academic researchers and regulators is crucial.
The communication framework presented in the figure below (Figure 1) outlines the information flow between different stakeholders in the regulatory system. Regulatory agencies are key players that act as licensing and supervisory authorities at national (NCAs) or European (EMA) levels, and generate and convey guidance on how to design successful drug-development programmes using specific channels to reach the main stakeholders in academia, namely, clinical researchers in university medical centres and hospitals. Other key stakeholders include research institutes, individual researchers working in drug R&D and the public and private funding bodies supporting these research activities. The figure was developed by the STARS consortium and published in the article “Strengthening regulatory science in academia: STARS, an EU initiative to bridge the translational Gap” (Starokozhko et al., 2020).
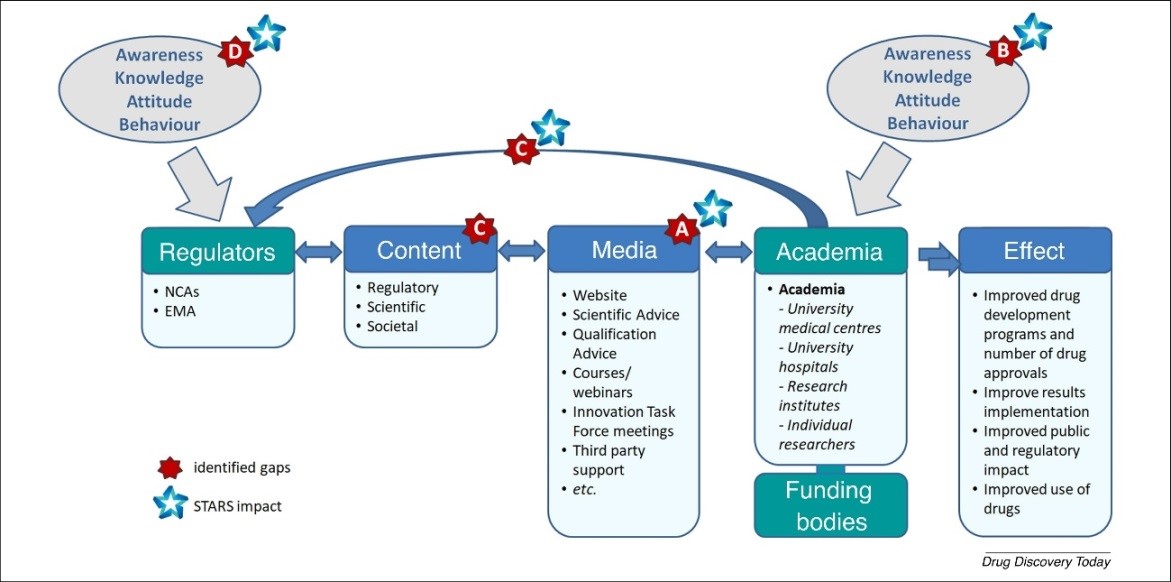
Figure 1. Information flow and identified gaps in the regulatory communication framework (cf. STARS White Paper by Starokozhko et al., 2020).
STARS has the objective and the potential to complement the gaps outlined on the figure above (Figure 1), to coordinate and to harmonise regulatory efforts among Member States and at European level to support academic health research for the benefit of patients.
The aim is to reach academic researchers very early in the planning of clinical research projects and relevant grant applications. A further aim is to strengthen regulatory knowledge in general by reaching clinical scientists during professional training and qualification.
Further information and current activities can be found in the STARS webpage.
Different activities have been carried out through the following work packages (WP):
- WP1: Management and Coordination of the project.
- WP2: Analysis of existing support activities in order to elaborate a Comprehensive inventory. After the finalization of this WP, STARS has launched a Comprehensive Inventory of existing support activities based on a detailed analysis of the currently established programmes. The inventory assists European academic drug developers in finding support on regulatory affairs.
- WP3: analyse capacities and services offered by the NAs and EMA.
WP4: Performance of three pilots:
Pilot I: transfer a best practice example of a training programme.
Pilot II: to establish a new support activity by addressing a gap in regulatory knowledge.
Pilot III: to implement a Comprehensive Curriculum to ensure harmonized tutorials addressing regulatory and scientific content.
- WP5: White paper and Final Report
- WP6: Dissemination and communication, workshops with the stakeholders and conferences
Finally, STARS will deliver consensual recommendations ensuring sustainable support of academic research and will propose additional support mechanisms based on a comprehensive analysis of needs. All activities will feed into a Common Strategy, which is a major roadmap to strengthen regulatory sciences knowledge.
The AEMPS through the Office for support of innovation and knowledge of medicinal products is leading the Pilot II which will be carried out during September in Spain exclusively.
Pilot II: AIM and PROCEDURE of STARS Pilot II: New support activity
Pilot II is one of three pilots in the EC-funded STARS project and represents a new support activity for strengthening regulatory knowledge in academia. The development of the novel support activity was based on a comprehensive survey data that have been collected within the course of the STARS project (WP2 and WP3). Different stakeholders were surveyed regarding different aspects on regulatory awareness, knowledge and support. In total, we analyzed data from 449 academic health research groups, 88 health research centres, 40 funding bodies and 21 NCAs with a view to best practices and gaps in the level of regulatory awareness, knowledge and approaches of the stakeholders in relation to academic clinical research and regulatory science.
Comprehensive data analysis revealed that there is a need in the academic community for tools to improve the communication between regulators and academia and that timely response is probably the most remarkable need.
Based on this finding, Pilot II was developed as a one-stop-shop platform where academic researchers will not only be able to find general content on regulatory aspects, but also to send some quick questions or queries.
The objective of this platform is to facilitate informal exchange of information and regulatory guidance in the development process, complementing and reinforcing existing formal regulatory procedures (like the National Scientific Advice and Innovation Offices). This service is free of charge.
To use this service you can contact AEMPS by filling the contact sheet form or by email (stars_pilot2@aemps.es). Please see further details in the Communication Board.
Within the one-stop-shop platform, the following boards are included:
- Explanation Board. General background information about the STARS project and about this pilot project.
- Information Board. It will include multiple regulatory documents and regulatory and scientific requirements in order to facilitate the development of medicinal products and to obtain the Marketing Authorization in the future. Thus, researchers can get all regulatory relevant information in just «one stop». Researchers will be able to look at and solve their queries. If the queries are not solved by digging into the information shared, researcher can use the Communication Board to directly contact AEMPS (see Communication Board).
- Communication Board. Here, researchers will find the contact details in order to get in contact with AEMPS (via contact sheet or email (stars_pilot2@aemps.es).
- Feedback Board. The researcher’s feedback is very important to evaluate the success of Pilot II. In the Feedback Board, we will ask the participants to fill a short questionnaire.
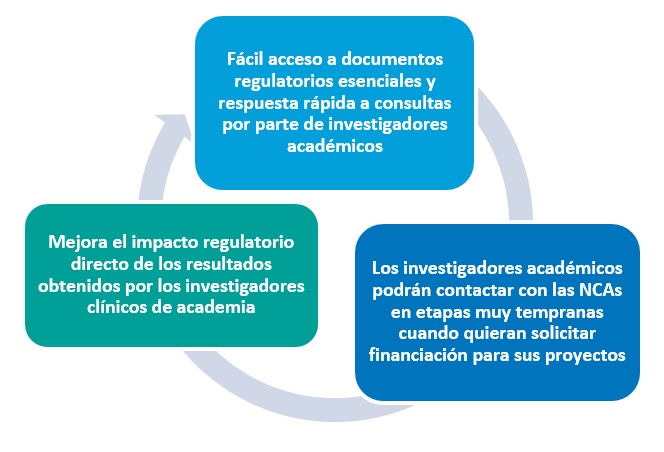
Figure 2. The concept of STARS Pilot II.
Invitation letter to take part in the Pilot II.
AIM and PROCEDURE of STARS Pilot III
Implementing the Comprehensive Curriculum
Pilot III is one of the three pilots in the EU-funded STARS project and represents the implementation of the Comprehensive Curriculum for strengthening regulatory knowledge in academia. It is planned and performed by AEMPS (Spanish Agency of Medicines and Medical Devices) in cooperation with PEI (Paul Ehrlich Institute, Germany) and OGYEI (National Institute of Pharmacy and Nutrition, Hungary), and supported by DLR/ Project Management Agency (German Aerospace Center, Germany).
The concept of Pilot III
The rapid expansion of pharmaceutical and biomedical products and increasing complexity of innovative technologies and products tend to start in non-commercial research institutions. To bridge the translational gap, highly skilled professionals need to be trained in regulatory knowledge to conduct research in compliance with complex regulatory policies and challenging procedures.
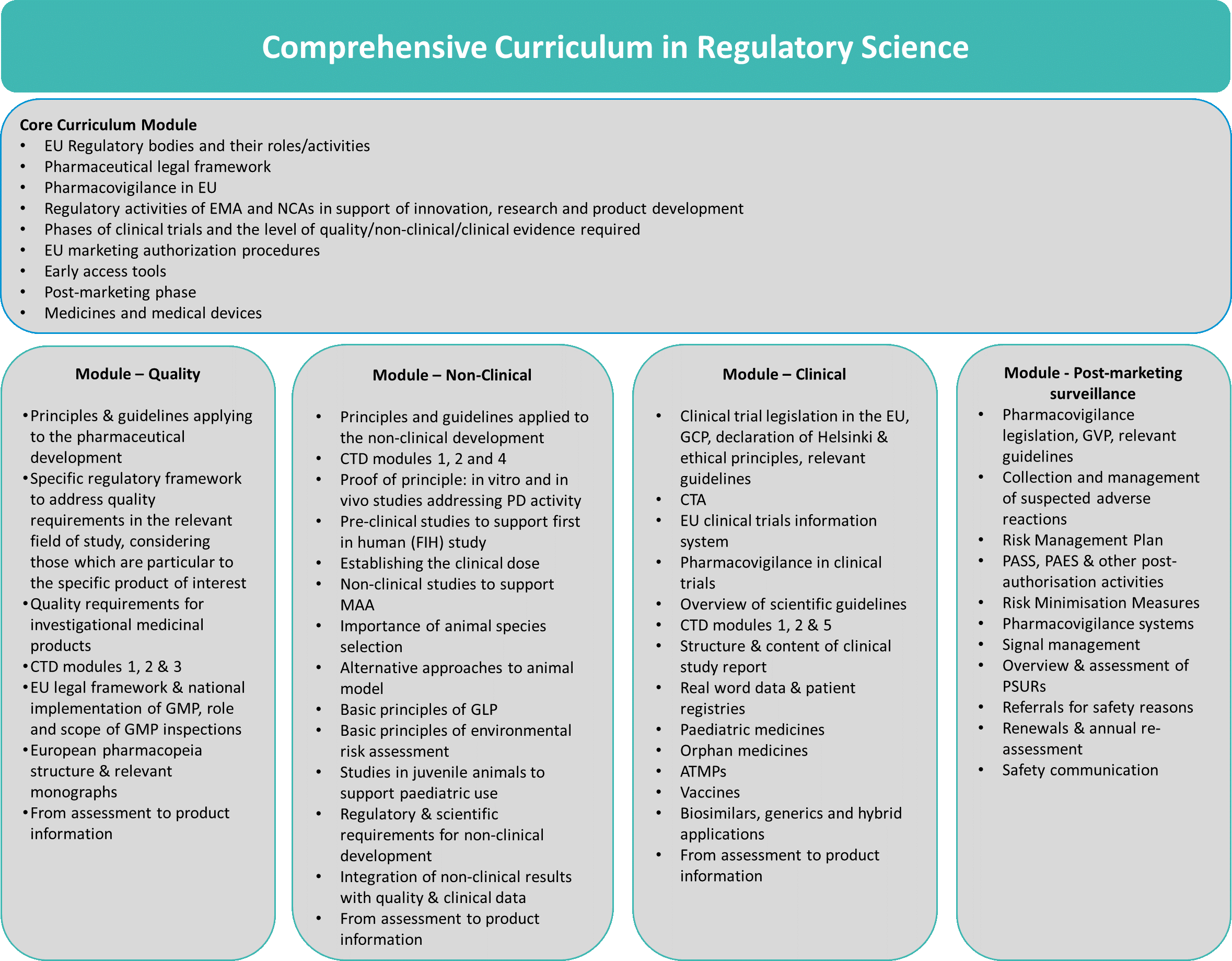
The STARS Comprehensive Curriculum (CpC) consists of five modules: Core Curriculum module and four more modules; Quality module, Non-clinical module, Clinical module and Post-marketing Surveillance module. All modules provide decisive information and knowledge to develop especially innovative medicinal products alongside the regulatory requirements to bring high quality, safety, and efficacy medicinal products to the European market.
Taking into account the time constraint for the project, Pilot III focuses on the STARS Core Curriculum module, giving some information on the topics included in the other modules (see Figure 1).
The format of the Pilot III will be based on a presentation called “Regulatory Support to Spanish Academia from STARS Core to Comprehensive Curriculum” with the aim of extending it in the future including all the points detailed on each of the modules.
The content of the Core Curriculum gives an overview of regulatory requirements at a general level, introducing the four special modules focusing on each of the above-mentioned areas throughout the whole medicinal product life cycle. This specialised education is a key entrance tool for clinical researchers and scientists to understand the legal regulatory requirements, to get familiar with the specific guidelines alongside the whole product life cycle and to learn about the timely use of the NCA support activities during the development of their products (Figure 1).
The overarching goal for the researchers is to successfully translate research into clinical development of medicinal products to reach as soon as possible to patients.
Figure 1. STARS Comprehensive Curriculum in regulatory Science.
Pilot III is provided free of charge. When participating attendees agree to answer a brief survey for the evaluation of Pilot III.In case of any queries about the Pilot feel free to contact us: stars_pilot3@aemps.es.
Pilot opens from February 16th to March 16th 2022.
Click Here:
https://www.csa-stars.eu/Pilot-III-Implementing-the-Comprehensive-Curriculum-1761.html


 What is the AEMPS contribution within the STARS project? – Pilot II
What is the AEMPS contribution within the STARS project? – Pilot II