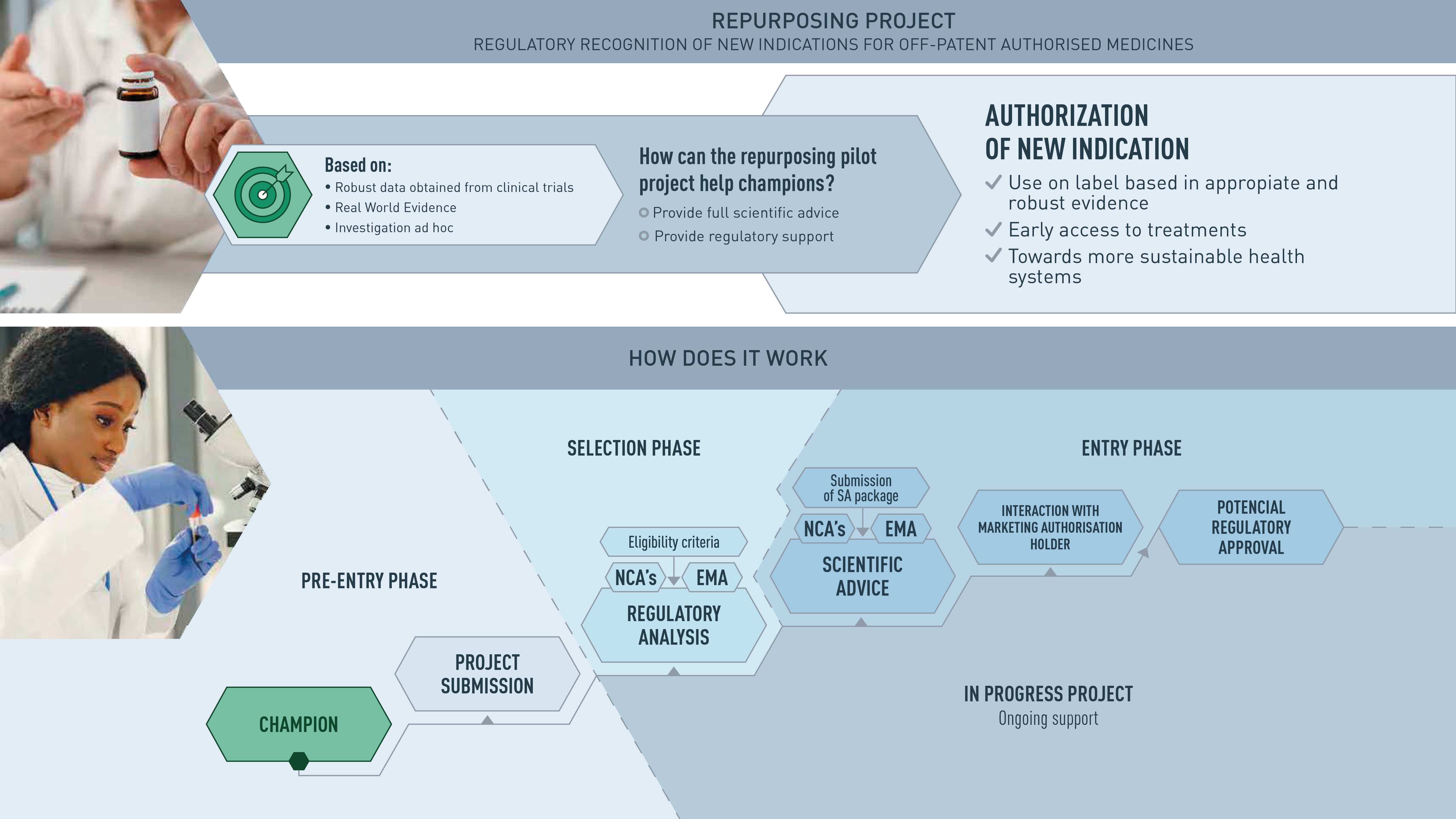
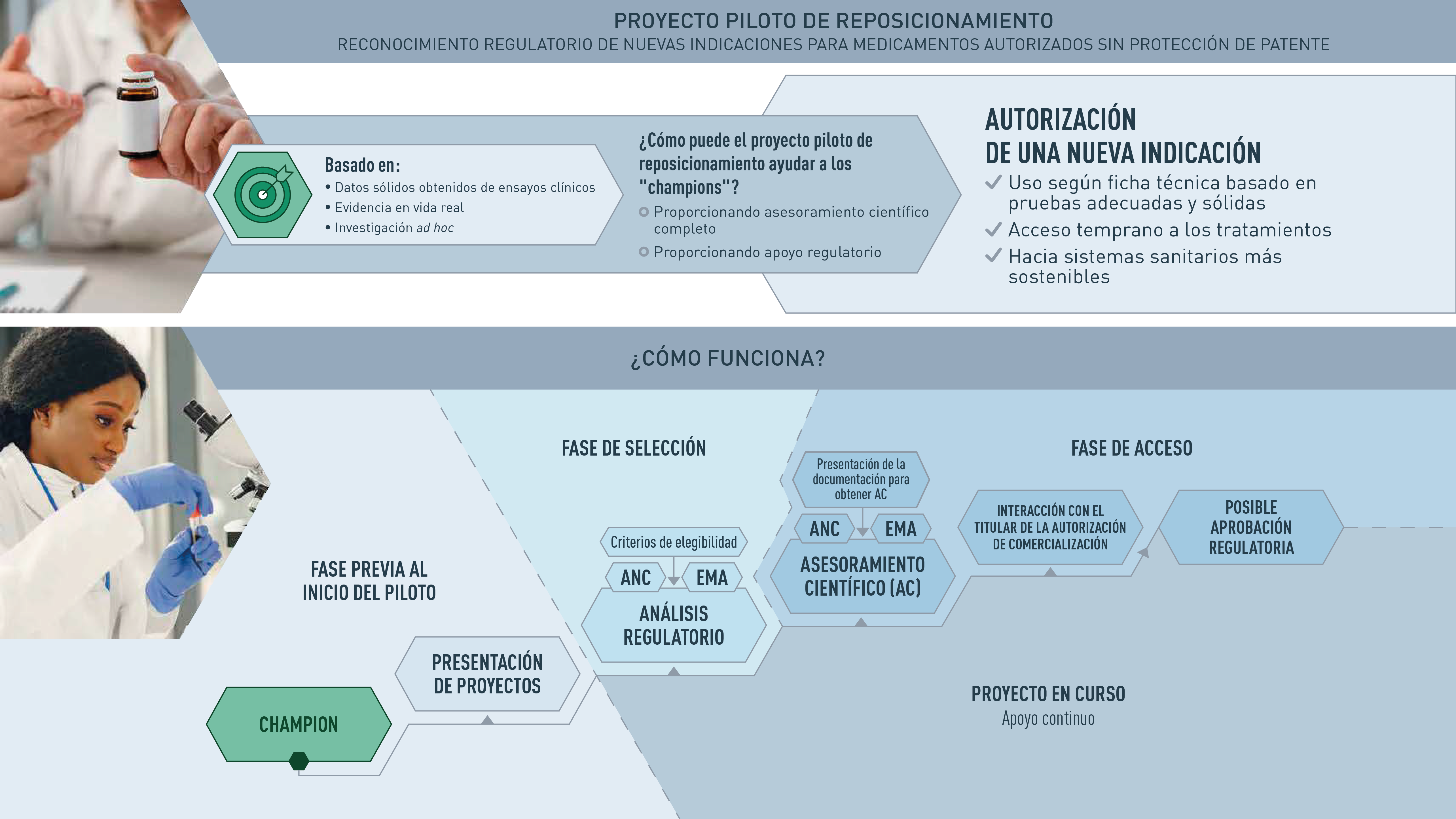
The AEMPS participates and leads the Repurposing project pilot, launched by the Heads of medicines Agency (HMA), the European Medicines Agency (EMA) and the European Commission (EC). This project was born as a result of discussions held within the EC Safe and Timely Access to Medicines for Patients group (STAMP), focused on proposing a framework for the repositioning of medicines.
The main objective of the Repurposing pilot project is to promote the repositioning of drugs among non-commercial sponsors (non-profit organizations, academic institutions and research groups -sponsors-) and to support these actors in the generation of sufficient evidence for a drug already known to be used off-label to support a future authorization application by the pharmaceutical company. In other words, the aim is for the regulatory authorities to lend their support to generate robust evidence for those drugs that are already authorized and have a routine use that is not included in the technical data sheet, and which could also be authorized for that indication. This initiative would be an important tool for offering therapeutic alternatives to patients and one of the levers of change for a healthy pharmaceutical ecosystem.
Candidate drugs to be part of this pilot must meet the following requirements:
- Contain a well-known and established active ingredient.
- Be an authorized drug (containing such active ingredient) outside of data and market exclusivity, as well as being off-patent or off-supplementary protection certificate.
- Being a drug intended for a different disease indication than the one for which it is currently authorized.
- It is intended for an indication in an area that is expected to have important benefits for public health; it treats a disease for which there are no or few currently authorized drugs available, or a disease that is associated with high morbidity and mortality despite the drugs that are already available.
- It must not be a drug whose repositioning responds to treatments for COVID-19 (in these cases they must follow the mechanisms and procedures established for them).
The pilot is open to academic and non-profit organizations (organizations and individuals) that have a specific interest in repositioning authorized drugs in a new indication for public health, have a scientific rationale for their repositioning program, and need scientific advice from a regulatory authority.
Sponsors wishing to participate in the pilot and submit their application to the EMA should complete the application form and send it to innov_spain@aemps.es by February 28, 2022.
The EMA has organized a webinar on February 17 so that all academic and non-profit organizations interested in the project can participate and ask questions. All the information is available here. In addition, you can consult this question and answer document.
The results have been included in the European repurposing pilot report.