From January 31st 2023 all new clinical trial applications must be submitted via European system CTIS.
Every clinical trial application processed through CTIS will have a valid final decision that the sponsor will receive via CTIS in accordance with Regulation 536/2014, without the requirement of additional national resolutions. In any case, an Appeal for Reconsideration may be brought to Head of the Spanish Agency of Medicines and Medical Devices within one month, according to Law 39/2015 (articles 123 and 124), October 1st, on the Common Administrative Procedure of Public Administration, or to interpose Contentious-Administrative Appeal to Contentious-Administrative Madrid Central Court, within two months from the next day of the reception of the decision notification, according to Contentious-Administrative Jurisdiction Regulation of July 13th 1998, without prejudice to any other appeal that may be interposed.
On July 31st 2021, the European Commission published the Commission Decision (EU) 2021/1240, of July 13th 2021, on the compliance of the EU portal and the EU database for clinical trials of medicinal products for human use.
Therefore, full application of Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use in the European Union and European Economic Area (EEA) – Iceland, Liechtenstein and Norway – took place on January 31st 2022.
The following sections gather further information related to the application of the Clinical Trials Regulation:
CTIS is the only way to send information about clinical trials in the EU and EEA from January 31st 2023. CTIS is a workspace for clinical trial sponsors and for competent authorities, and also a clinical trial public website.
Countries from EU and EEA will assess and supervise clinical trials in CTIS, while European Medicines Agency (EMA) will establish and execute maintenance work for CTIS website. The European Commission will assure the correct interpretation and implementation of the Clinical Trials Regulation.
The objective of the regulation is to create a good environment to perform clinical trials in Europe and, at the same time, to maintain high quality standards where participant´s safety is assured.
Application of this regulation means:
- Same standards to perform clinical trials within the EEA.
- Same clinical trial scientific documentation (Part I) for all European countries and countries from the EEA, in addition to Part II documentation which differs by each member state.
- Better efficiency in the clinical trial approval process.
- A single submission of the clinical trial application via CTIS to every concerned country.
- A coordinated assessment procedure of part I by concerned member states, led by one of them (Reporting Member State) in a mutual recognition procedure.
- Single decision principle. For each member state, the final decision will be composed of an opinion from a competent authority and an ethics committee.
- Clear evaluation deadlines and tacit approval principle (for the member state) or withdrawal (for the sponsor) in case the deadline has expired.
- Higher transparency: clinical trial documentation, assessment details and results summary for lay people.
CTIS will support EU member states, EEA countries and sponsors working processes, during the whole clinical trial life cycle. CTIS will also give regulatory oversight and tools for clinical trial monitoring:
- Tools for the sponsor to send initial applications for the authorisation of a clinical trial, substantial modifications or requests to add one or more member states.
- Assessment.
- Clinical trials safety monitoring once authorised.
- Notifications.
- Corrective measures.
- Safety tracking.
- Data update for the accurate clinical trial monitoring.
- Clinical trials update (non-substantial modifications notification).
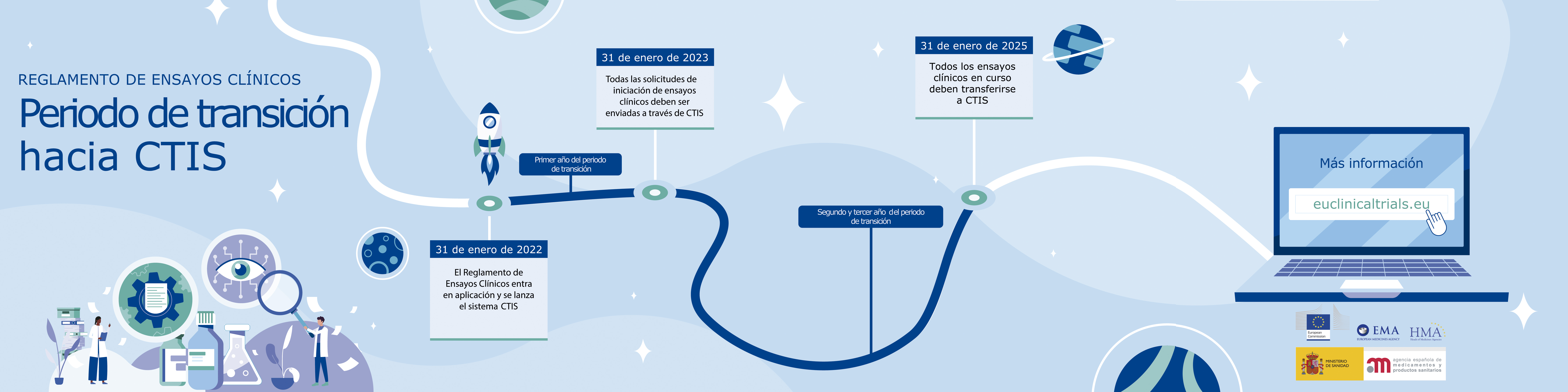
Relevant dates in this transition period:
- From January 31st 2022 to January 31st 2023: every clinical trial initial application can be submitted according to CT Regulation or national legislations derived from Directive 2001/20/EC.
- From January 31st 2023: every clinical trial initial application must be submitted according to CT Regulation via CTIS.
- From January 31st 2023 to November 31st 2024: ongoing clinical trials already authorised can remain operating by previous legislation or change to the new one by submitting a transition application via CTIS. Sponsors must take into account that the transition period goes from 60 to 106 days so they will have to apply for transition soon enough in case clarifications are needed.
- On January 31st 2025 all ongoing clinical trials must be transitioned and operating by current CT Regulation with its authorization on CTIS.
European Medicines Agency (EMA), in cooperation with all member states, has developed an online training program, approaching the whole clinical trial life cycle from application to authorization and clinical trial monitoring. These programs are designed to help clinical trials sponsors, national competent authorities, ethics committees, European Commission and EMA staff using Clinical Trials Information System (CTIS).
AEMPS will update this website content according to available information.
Any related question can be addressed to AEMPS Clinical Trials Division (aecaem@aemps.es).
In case the question cannot be solved there, AEMPS staff will send it to the proper recipient so there is no need to send the same question several times.

